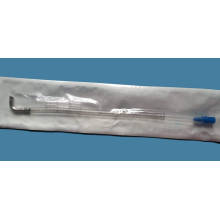
CE Venous Cannula Without Connector
Basic Info
Model No.: kx0201
Product Description
Model NO.: kx0201 Material: Plastic Ethylene Oxide Sterilization: Ethylene Oxide Sterilization Group: Adults and Children Size: 12fr-40fr Specification: CE, ISO 13485, ISO 9001 GMP HS Code: 9018390000 Type: Catheter Certification: CE, SGS, ISO13485 Quality Guarantee Period: Two Years Logo Printing: With Logo Printing Trademark: Qunxing Origin: China Application: In cardiac operation under direct vision, it can be inserted into the vein for blood flow in extracorporeal circulation.
Features:
1. Flexible, kink resistant wirewound body;
2. Thin wall PVC construction;
3. Tapered, multi-port bullet tip for easy insertion and optimum flow rates;
4. Single stage;
5. Angled tip
Model No.: KX0202
Sizes: 12FR 14FR 16FR 18FR 20FR
22FR 24FR 26FR 28FR 30FR
32FR 34FR 36FR 38FR 20FR
Contact us if you need more details on Disposable Medical. We are ready to answer your questions on packaging, logistics, certification or any other aspects about Cardiac Surgery、Cannula. If these products fail to match your need, please contact us and we would like to provide relevant information.
Features:
1. Flexible, kink resistant wirewound body;
2. Thin wall PVC construction;
3. Tapered, multi-port bullet tip for easy insertion and optimum flow rates;
4. Single stage;
5. Angled tip
Model No.: KX0202
Sizes: 12FR 14FR 16FR 18FR 20FR
22FR 24FR 26FR 28FR 30FR
32FR 34FR 36FR 38FR 20FR
| MODEL NO. | SPECIFICATION | OUTER DIAMETER (MM) | CONNECTION SITE |
| KX0201-12 | 12FR | 4.0 | 1/4' ' |
| KX0201-14 | 14FR | 4.7 | 1/4' ' |
| KX0201-16 | 16FR | 5.3 | 1/4' ' |
| KX0201-18 | 18FR | 6.0 | 1/4' ' -3/8' ' |
| KX0201-20 | 20FR | 6.7 | 3/8' ' |
| KX0201-22 | 22FR | 7.3 | 3/8' ' |
| KX0201-24 | 24FR | 8.0 | 3/8' ' |
| KX0201-26 | 26FR | 8.7 | 3/8' ' |
| KX0201-28 | 28FR | 9.3 | 3/8' ' |
| KX0201-30 | 30FR | 10.0 | 3/8' ' |
| KX0201-32 | 32FR | 10.7 | 3/8' ' |
| KX0201-34 | 34FR | 11.3 | 3/8' ' |
| KX0201-36 | 36FR | 12.0 | 1/2' ' |
| KX0201-38 | 38FR | 12.7 | 1/2' ' |
| KX0201-40 | 40FR | 13.3 | 1/2' ' |
Product Categories : Venous Cannulae
Other Products
Hot Products
High Quality Oxygen Concentrator with Ce ISO (SC-SF-3AW)Ultrasonic, Ultrasound Scanner Black White Doppler Laptop Portable (SC-ECO3 expert)Ultrasound Color Scanner Doppler Laptop Portable (SC-ECO5)Touchscreen Animal Vet ECG EKG Veterinary Monitor with FDA (V-C50)Vital Signs Monitor Transport Touchscreen Patient Monitor (SC-NC3)12.1 Inch Modular Patient Monitor Touch Screen Ccu ECG EKG Machine Telemetry (SC-C100)Veterinary Urine Analyzer with Printer Veterinary Chemical AnalyzerPortable Veterinary Anesthesia Machine, Veterinary Ventilator Scam-1 VetVeterinary Infusion System Pump Syringe Pump with Ce (SC-801vet)Pediatric Infrared Vein Illuminator Vein Detector Vein Finder (SC-B300)Animal Veterinary Ultrasound Color Doppler Laptop Veterinary DopplerUltrasound Doppler Twins Touchscreen Fetal Monitor with Ce (SC-STAR5000D)Table Top Portable Fetal Doppler Ultrasonic Ultrasound (SC-FHD02)12.1inch Fetal Maternal Monitor Modular Touchscreen Monitor Obstetric Fetal Doppler Ultrasound Ce Approved (SC-STAR5000F)Transport Emergency Transfer Patient Monitor Touchscreen Handheld Ambulance Vital Signs Monitor Sc-C30Neonatal Patient Monitor Newborn Infant Nicu Touch Screen Vital Signs Monitor Apnea Monitor FDA Approved (SC-C60)